Food drug administration 10903 new hampshire avenue silver spring md 20903 www fda gov regenerative medicine aka stem cell therapy treatments availabl e.
Fda approved stem cell therapies 2019.
While that s a compelling reason to bank cord blood it is important to understand what these treatments are and how stem cells from your child s.
Continues to warn patients of the risk of unapproved stem cell.
Stem cells from a newborn s umbilical cord blood are fda approved to treat over 80 diseases helping regenerate the body after chemotherapy radiation and other aggressive medical procedures.
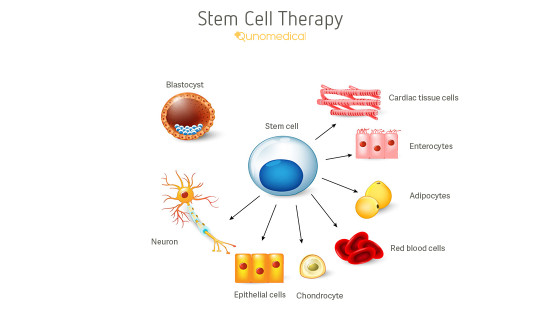
The only stem cell based products that are fda approved for use in the united states consist of blood forming stem cells hematopoietic progenitor cells derived from cord blood.
Fda sends warning to company for selling unapproved umbilical cord blood and umbilical cord products that may put patients at risk.
Currently the only stem cell products that are fda approved for use in the united states consist of blood forming stem cells also known as hematopoietic progenitor cells that are derived from.
The fda is offering doctors a new path to testing stem cell therapies for clinics to prove the safety and efficacy of their fat derived stem cell treatments to the fda they must run rigorous.
The immediate appeal of americord s services lies in the amazing strides physicians and scientists have made since the first successful stem cell transplant.